Third-party Reproduction: Sperm, egg, and embryo donation and surrogacy
INTRODUCTION
The phrase “third-party reproduction” refers to the use of eggs, sperm, or embryos that have been donated by a third person (donor) to enable an infertile individual or couple (intended recipient) to become parents. Donors may be known or anonymous to the intended recipient. “Third-party reproduction” also includes traditional surrogacy and gestational carrier arrangements. Traditional surrogacy refers to a treatment in which a woman is inseminated with sperm for the purpose of conceiving for an intended recipient. The surrogate in this scenario has a genetic and biological link to the pregnancy she might carry. In contrast, a gestational surrogate (also called a gestational carrier [GC] or uterine carrier) is an individual in which embryos created by the intended parents are transferred into the surrogate’s uterus, which has been prepared hormonally to carry a pregnancy. The gestational surrogate has no genetic link to the fetus she is carrying. Traditional surrogacy arrangements often are perceived as controversial with the potential to be complicated both legally and psychologically. Despite the requirement for in vitro fertilization (IVF) to create embryos, the utilization of a gestational surrogate, legally, is a lower-risk procedure and is the more common approach conducted in the United States.
Third-party reproduction is a complex process requiring consideration of social, ethical, and legal issues. The increased use of egg donation has required a reconsideration of the social and ethical impact this technology has had on prospective parents, their offspring, and the egg donors themselves. Surrogacy has been acknowledged within the reproductive-medicine community as well as by the American Society for Reproductive Medicine (ASRM). Surrogacy arrangements nevertheless remain controversial and are subject to both legal and psychosocial scrutiny. This booklet will discuss the options for third-party reproduction, reviewing sperm donation, egg donation, embryo donation, and both traditional surrogacy and gestational surrogacy.
EGG DONATION
The first pregnancy achieved with egg donation was reported in 1984. Since that time, there has been increasing use of egg donation to help infertile couples/individuals conceive. Egg donors are identified, and, through the process of IVF, eggs are obtained from the donor’s ovaries and donated to the intended recipient. Sperm obtained from the recipient’s partner (or a sperm donor) is used to fertilize these eggs, and embryos are transferred into the recipient’s uterus. If pregnancy occurs, the recipient will have a biological but not genetic relationship to the child; her partner (if he provided the sperm) will be both biologically and genetically related.
Indications for Egg Donation
Egg donation initially was intended for women with ovarian failure. Typically, women were prematurely menopausal as a result of disease, chemotherapy, radiation therapy, or surgical removal of their ovaries. Egg donation is appropriate for women who were born without ovaries. Due to the success of the procedure, as well as the improvements in IVF technology, these indications have been expanded. Egg donation may be offered to women who are known to be affected by or be the carrier of a significant genetic disease who would prefer not to pass this disease on to her offspring. This indication includes women who have a significant family condition where their carrier status cannot be determined. Normal-ovulatory women who appear to have an egg factor as the cause of their infertility often are candidates for egg donation. In many instances, this includes women with multiple failures to conceive after IVF, women of advanced reproductive age, and women with inadequate response to ovulation induction.
Who are Egg Donors?
There are several ways of obtaining donor oocytes (eggs).
Anonymous donors: Women who are not known to the recipient. Donors may be recruited through established egg donation programs or may be identified through agencies.
Known or directed donors: Women who are known to the recipient. The donor is generally a close relative or friend. In some instances, recipients advertise directly for donors in newspapers or on the Internet. In these circumstances, the recipient couple and the donor are known to each other in a limited way, having met without an intermediary program or agency. Recipients should exercise caution about recruiting donors directly without having an intermediary program or agency screen donors or without seeking legal counsel.
IVF programs: Women undergoing IVF may agree to donate their excess eggs to infertile patients. This source of donors is limited, probably because of the perceived coercive nature of the donation, particularly if the women are offered a financial discount on their own IVF cycle.
Evaluation of the Egg Donor
All donors, both anonymous and known, should be screened according to the most recent guidelines recommended by the ASRM. Donors should have attained their state’s age of legal majority and preferably should be between the ages of 21 and 34. The rationale for the lower age limit is to ensure that the donor is mature enough to provide true informed consent. The rationale for the age of less than 34 is that younger women typically respond favorably to ovulation induction, produce more eggs and high-quality embryos with high implantation, and have subsequent higher pregnancy rates than older women. If the donor is over the age of 34, recipients should be informed as to the cytogenetic risk of having a child with a chromosomal abnormality such as Down syndrome and the impact of donor age on pregnancy rates.
Both anonymous and known donors should complete an extensive medical questionnaire that details their personal and family medical history. Included in this questionnaire should be a detailed sexual history, substance abuse history, history of family disease, and psychological history. In the United States, the Food and Drug Administration (FDA) requires that all egg donors be screened for risk factors for, and clinical evidence of, communicable infections and diseases. A donor is ineligible if either screening or testing indicates the presence of a risk factor for, or clinical evidence of, a communicable infection or disease. For anonymous donors, the questionnaire should assess the donor’s motivation for donating her eggs and provide insight into the donor personality, her hobbies, educational background, and life goals. This document ultimately will be shared with the recipient and provides her with insight into a donor she will never meet. A medical professional reviews this history with the donor and conducts a comprehensive physical examination.
Generally, each donor completes a written psychometric test prior to meeting with a mental health professional (MHP). In addition to reviewing the psychometric test, the MHP has the opportunity to further evaluate the donor, discuss the many complex ethical and psychosocial issues she may encounter, and confirm the donor truly is able to provide informed consent for egg donation.
The laboratory testing of all donors should include screening and testing for syphilis, hepatitis B and C, human immunodeficiency virus (HIV)-1 and HIV-2, Neisseria gonorrhoeae, and Chlamydia trachomatis, as well as screening for human transmissible spongiform encephalopathy and testing when risk factors for it exist. All infectious-disease testing must be done and noted to be negative within 30 days before egg donation. Donors also should have documentation of their blood type and Rh status, complete blood count, and rubella titer. Genetic screening of donors should be based on ethnicity. All donors should be tested for the presence of a cystic fibrosis (CF) mutation. Donors of Asian, African, and Mediterranean descent should undergo a hemoglobin electrophoresis as a screen for sickle cell trait and thalassemias. If the donor is of Ashkenazi Jewish origin, CF mutation analysis and screening for Tay-Sachs disease, Canavan disease, familial dysautonomia, Gaucher disease, and other genetic diseases is indicated. Donors who are of French Canadian descent should be screened for CF as well as Tay-Sachs disease. Additional genetic testing and karyotyping of the donor is not required but may be offered by individual programs as part of their standard procedure or upon the request of the recipient couple.
Evaluation of the Recipient Couple
Evaluation of the recipient couple is similar to that of couples undergoing routine IVF. The physician should obtain a comprehensive medical history from both partners. In addition, the female assessment will include a comprehensive gynecologic history and complete physical exam. From a laboratory perspective, the female should have an assessment of ovarian reserve, when appropriate, blood type and Rh, and rubella and cytomegalovirus (CMV) testing. A Pap smear and cultures for Neisseria gonorrhoeae and Chlamydia trachomatis should be obtained.
The female partner should have an evaluation of her uterine cavity with a hysterosalpingogram (HSG), sonohysterogram (SHG), or hysteroscopy. If the female recipient is over the age of 45 years, a more thorough evaluation with assessment of cardiac function, risk for pregnancy-induced hypertension, and gestational diabetes should be considered. A high-risk obstetrical consultation is recommended to discuss the impact of advanced maternal age on pregnancy, as well as any medical conditions that may impact a pregnancy. The male assessment will include a semen analysis, blood type and RH factor, and genetic testing as indicated. The intended recipient couple should be screened for syphilis, hepatitis B and C, HIV-1, and HIV-2.
Preparation of the Donor for Egg Retrieval
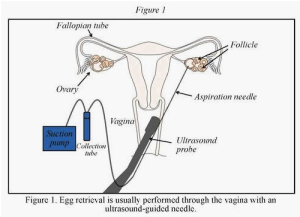 In order to retrieve multiple eggs from the donor’s ovaries, the donor must be given a combination of hormonal medications to stimulate the development of multiple eggs within the ovary. This technique is called ovulation induction. The medications may include a gonadotropin-releasing hormone agonist (GnRH-a) or gonadotropin-releasing hormone antagonist (GnRH-ant) to prevent the donor from spontaneously ovulating, and either human menopausal gonadotropin (hMG) or recombinant follicle-stimulating hormone (r-FSH), both of which are able to induce egg development. Development of eggs is monitored by ultrasound and measurement of hormones in blood. When the egg development is at the appropriate size, ovulation is triggered by an injection of human chorionic gonadotropin (hCG).
In order to retrieve multiple eggs from the donor’s ovaries, the donor must be given a combination of hormonal medications to stimulate the development of multiple eggs within the ovary. This technique is called ovulation induction. The medications may include a gonadotropin-releasing hormone agonist (GnRH-a) or gonadotropin-releasing hormone antagonist (GnRH-ant) to prevent the donor from spontaneously ovulating, and either human menopausal gonadotropin (hMG) or recombinant follicle-stimulating hormone (r-FSH), both of which are able to induce egg development. Development of eggs is monitored by ultrasound and measurement of hormones in blood. When the egg development is at the appropriate size, ovulation is triggered by an injection of human chorionic gonadotropin (hCG).
The eggs are harvested from the ovary approximately 34-36 hours after hCG administration through a process called transvaginal ultrasound aspiration (Figure 1). This is done by placing a transvaginal ultrasound probe, which has a needle guide, into the vagina. A needle is placed into this guide, through the vaginal wall, and into the ovary. The eggs are obtained, evaluated for maturity, and then are inseminated with the male partner’s sperm (donor sperm also may be used), which has been processed in the laboratory. For further details regarding the types of ovulation induction medications and the IVF procedure, please refer to the ASRM patient information booklet titled Assisted Reproductive Technologies.
Preparation of the Recipient for Embryo Transfer
 In order for embryos to implant into the recipient’s uterus, the endometrium (uterine lining) must be prepared and synchronized with the donor reproductive cycle. Numerous methods of endometrial preparation have been described; however, the principle of hormonal preparation is similar. Women who have ovarian function are given a GnRH-a to temporarily suppress their menstrual cycle. When the donor starts her hormonal medications to stimulate her ovaries, the recipient is given estradiol to stimulate the endometrium to develop. Estradiol may be given in the form of an oral pill, transdermal patch, or injection. Ultrasound assessment of the endometrium and blood tests may occur during this time. The recipient begins progesterone on the day after the donor receives hCG. Progesterone causes specific maturational changes within the endometrium that enable the embryo to implant. Progesterone may be given by intramuscular injection, vaginal gel, or tablet.
In order for embryos to implant into the recipient’s uterus, the endometrium (uterine lining) must be prepared and synchronized with the donor reproductive cycle. Numerous methods of endometrial preparation have been described; however, the principle of hormonal preparation is similar. Women who have ovarian function are given a GnRH-a to temporarily suppress their menstrual cycle. When the donor starts her hormonal medications to stimulate her ovaries, the recipient is given estradiol to stimulate the endometrium to develop. Estradiol may be given in the form of an oral pill, transdermal patch, or injection. Ultrasound assessment of the endometrium and blood tests may occur during this time. The recipient begins progesterone on the day after the donor receives hCG. Progesterone causes specific maturational changes within the endometrium that enable the embryo to implant. Progesterone may be given by intramuscular injection, vaginal gel, or tablet.
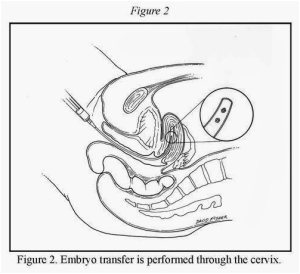
Embryos are transferred into the recipient’s uterus, usually within three to five days after the eggs are fertilized in the laboratory. The embryo transfer (Figure 2) is performed by placing a small catheter with the embryos through the cervix and into the uterus. If the recipient couple has extra embryos, these embryos may be cryopreserved (frozen) for use at a later time in additional attempts to achieve a pregnancy.
The hormonal replacement regimen of estradiol and progesterone is continued until the recipient achieves a positive pregnancy test. If the pregnancy test is positive, estradiol and progesterone are continued through the first trimester to support the early pregnancy.
Pregnancy Rates with Egg Donation
The pregnancy rate with egg donation depends on many factors but is generally independent of the age of the recipient. Success rates compiled by the Centers for Disease Control (CDC) for the year 2009 show the average live-birth rate per fresh embryo transfer is 55.1% for all egg-donor programs. The major risk for egg-donor programs is multiple gestations. In 2009, of the 6,553 pregnancies conceived with egg donation, 5,595 resulted in a live birth. Of these, the multiple pregnancy rate was 39.9% with 37.1% being twins and 2.8% being triplets or greater. Because many of the pregnancies miscarry before the actual number of fetuses can be determined, the percentage of multiple pregnancies actually may be higher. The current trend is to reduce the number of embryos transferred in an effort to reduce the risk of multiple gestations. Most programs will limit the number of embryos transferred to two if the donor is between the ages of 21 and 34.
SPERM DONATION
Artificial insemination using donor sperm has been practiced for over a century, although the first published reports about the practice were in 1945. Over the past 10 years, the use of donor sperm has decreased as the use of intracytoplasmic sperm injection (ICSI) for the treatment of male infertility has become widespread. Since the late 1980s, with the emergence of acquired immunodeficiency syndrome (AIDS), artificial donor insemination has been performed exclusively with frozen and quarantined sperm. Current FDA and ASRM guidelines recommend that sperm be quarantined for at least six months before being released for use.
Indications for Sperm Donation
Currently, therapeutic-donor insemination (DI or TDI) is appropriate when the male partner has severe abnormalities in the semen parameters and/or reproductive system. These abnormalities include both obstructive (caused by a blockage of the ejaculatory ducts) and nonobstructive (due to testicular failure) azoospermia (absence of sperm), which may be congenital or acquired. Examples of obstructive azoospermia include congenital absence of the vas deferens or previous vasectomy. Examples of nonobstructive azoospermia include primary testicular failure or secondary testicular failure due to previous radiation treatment or chemotherapy. Severe oligospermia (decreased sperm count) or other significant sperm or seminal fluid abnormalities also are indications for DI. DI also is indicated if the male has ejaculatory dysfunction or if he is a carrier or affected with a significant genetic defect and would prefer not to pass this gene on to his children. DI may be used if the female is Rh-sensitized and the male partner is Rh-positive. DI often is used in the treatment for a single woman who desires a pregnancy but who lacks a male partner.
Selection of Sperm Donors
Sperm donors should be of legal age and ideally less than 40 years of age to minimize the potential hazards of aging. Traditionally, donors have been anonymous; however, the donor also may be known or directed to the couple or single woman. The ASRM believes it is important that both anonymous donors and donors known to the recipient–though not necessarily intimate sexual partners–undergo the same initial and periodic screening and testing process. However, the FDA only requires that anonymous sperm donors be screened for risk factors for, and clinical evidence of, communicable disease agents or diseases.
A donor is ineligible if either screening or testing indicates the presence of a communicable disease or of a risk factor for a communicable disease. A comprehensive medical questionnaire to evaluate the health of a donor and review his family medical history is the primary focus in selecting a donor. Particular attention is paid to the potential donor’s personal and sexual history to exclude those males who are at high risk for communicable disease including HIV, hepatitis, and other sexually transmitted diseases. A family medical health history is obtained for at least two generations of family members. Prospective donors then undergo a physical examination with screening for visible physical abnormalities, as well as testing for sexually transmitted diseases. Routine blood analysis includes documentation of the donor’s blood type. Current FDA regulations require infectious-disease testing to be performed and noted to be negative within 7 days of all sperm donations. The sperm are then collected by masturbation, concentrated into small volumes of motile sperm, and frozen until used. For anonymous donors, testing for Treponema pallidum (syphilis), Chlamydia trachomatis, Neisseria gonorrhoeae, HIV-1, HIV-2, human T-lymphotropic virus (HTLV)-I and HTLV-II, CMV, hepatitis B surface antigen, and hepatitis C antibody are performed prior to donation and thereafter should occur at six-month intervals, according to FDA guidelines. Although the FDA exempts directed sperm donors from the six-month retesting requirement, the ASRM recommends that directed donors be retested just as anonymous donors are retested. In contrast to the other communicable diseases, a positive CMV result does not make the sperm donor ineligible, since many programs allow his sperm to be used with CMV-positive recipients. Comprehensive genetic testing is impractical; however, ethnically based genetic testing is standard in most sperm banks.
It is recommended that all sperm donors, anonymous and directed, have a psychological evaluation and counseling by a MHP. The assessment should seek any psychological risks and evaluate for financial and emotional coercion. The donor should discuss his feelings regarding disclosure of his identity and plans for future contact. Psychological testing may be performed, if warranted.
Ideally, the sperm donor should undergo a semen analysis, and the test sample should be thawed to evaluate post-freezing/thawing semen parameters. Sperm susceptibility to damage with freezing varies among individuals, as well as among samples of a given donor. Donors are selected if the post-thaw semen parameters meet a minimum standard. In general, specimens should contain a minimum of 20 to 30 million motile sperm per milliliter after thawing. Post-thaw motility is generally in the range of 25% to 40%. There are two types of samples offered by most sperm banks. Intracervical insemination specimens (ICI) are prepared for intracervical inseminations only and must be washed if used for intrauterine inseminations. Although sperm preparations for ICI are available, the majority of reproductive endocrinology practices perform intrauterine insemination (IUI). IUI samples are pre-washed for intrauterine insemination. Both ICI and IUI semen samples are frozen and quarantined for a minimum of 180 days. They are not released until the donor is retested for communicable diseases and the results are negative.
In addition to the medical information that is obtained from the donor, donors are asked to provide detailed information about their personal habits, education, hobbies, and interests. Sperm banks may provide pictures of the donor and video or audiotapes from the donor. Donors may identify themselves as open to contact from any child conceived through DI once a child reaches legal age.
The Insemination Procedure
Before proceeding with donor insemination, a couple must be evaluated thoroughly for the causes of infertility with a comprehensive medical history and physical exam for both partners. It is recommended that the woman have documentation of ovulation with either an ovulation predictor kit or a basal body temperature chart. In addition to a pelvic examination, a hysterosalpingogram (HSG) or sonohysterogram (SHG) will evaluate further the uterine cavity and patency of the fallopian tubes.
Insemination may be timed based on a woman’s natural cycle or in conjunction with an ovulation induction cycle and should occur close to the time of ovulation. The procedure is relatively simple and is performed in the physician’s office. The woman is positioned on the examination table as if in preparation for a pelvic examination. The physician or nurse then places the speculum into the vagina to visualize the cervix. The semen sample is drawn up into an insemination catheter attached to a syringe. With IUI (Figure 3), the washed semen specimen is placed through the cervix and into the uterine cavity. This enables a higher concentration of sperm to reach the uterine cavity and fallopian tubes, which is where fertilization occurs.
Pregnancy Rates
The pregnancy rates with donor insemination depend on many factors. These include the age of the female recipient and the presence of other female fertility factors such as endometriosis, tubal disease, or ovulatory dysfunction. In general, the monthly chance of pregnancy ranges from 8% to 15%. A number of studies have demonstrated that the pregnancy rates with IUI are greater than ICI when frozen donor semen is used. The risk of birth defects as a result of conceiving with donor insemination is no different than natural conception and is in the range of 2% to 4%.
EMBRYO DONATION
Embryo donation is a procedure that enables embryos that were created by couples undergoing fertility treatment to be transferred to infertile patients in order to achieve a pregnancy. Indications for embryo donation include untreatable infertility that involves both partners, untreatable infertility in a single woman, recurrent pregnancy loss thought to be related to embryonic factors, and genetic disorders affecting one or both partners.
The process of embryo donation requires that the recipient couple undergo the appropriate medical and psychological screening recommended for all gamete donor cycles. In addition, the female partner undergoes an evaluation of her uterine cavity and then her endometrium is prepared with estrogen and progesterone in anticipation of an embryo transfer.
In the United States, embryo donation must meet established FDA guidelines for screening of the donors. In the case of embryos that have been created previously, the FDA recommends–but does not require–that the couple who created these embryos undergoes the requisite screening and testing required of all egg and sperm donors. For embryos that are created specifically for donation, the sperm donor and egg donor must be screened and tested as any other sperm and egg donors who are not intimate sexual partners of the recipients.
Embryo donation is a controversial process from both an ethical as well as a legal standpoint. What differentiates embryo donation from either egg or sperm donation is that the child born to the couple will have no genetic link with them, yet all parties will benefit from the biologic relationship they share through the commitment the parents have made to gestate this embryo. Of paramount importance is that informed consent and counseling be provided to both the donors of the embryos and the recipient couple to address all of the potential issues embryo donation might raise. In addition, due to the absence of explicit laws regarding embryo donation, couples should consult with legal counsel regarding the necessity of a pre-donation agreement as well as the necessity of seeking a judicial determination or recognition of parentage.
Pregnancy following embryo donation depends on the quality of the embryos that were frozen, the age of the woman who provided the eggs, and the number of embryos transferred. There are no national statistics on the pregnancy rate with embryo donation due to the limited number of embryo donation cases nationwide.
SURROGACY
Surrogacy is both a medically and emotionally complex process that requires careful evaluation by medical professionals, MHPs, and legal professionals to ensure that the procedure is satisfactory for both the surrogate as well as the intended parents. A surrogate is a woman who carries a pregnancy for another couple or woman. There are two types of surrogacy arrangements: traditional surrogacy in which the surrogate is inseminated with sperm from the male partner of the intended parent couple (donor sperm may be used as well) and gestational carrier (GC) in which the surrogate carries a pregnancy created by transferring an embryo created with the sperm and egg of the intended parents (donor sperm or donor eggs may be used as well). A GC has no genetic relationship to the child.
Much of the conflict surrounding surrogacy is a result of issues surrounding the legality of binding agreements agreed to prior to the conception or birth of a child. Traditional surrogacy is, therefore, an approach that carries more legal risk. As such, the majority of surrogacy conducted in the United States involves the use of a gestational carrier.
Indications for Use of a Gestational Carrier
The initial indication for use of a GC is a woman who has normally functioning ovaries but who lacks a uterus. Women with congenital absence of the uterus (müllerian agenesis) or prior hysterectomy due to benign or malignant conditions are obvious candidates. Women with congenital müllerian anomalies such as a T-shaped or hypoplastic uterus with a history of infertility or repetitive pregnancy loss also are candidates, as are women with untreatable intrauterine scar tissue. A gestational carrier also is an appropriate treatment for women with a medical contraindication to pregnancy. Examples of medical conditions that may prompt the use of a gestational surrogate include severe heart disease, systemic lupus erythematosis, history of breast cancer, severe renal disease, cystic fibrosis, severe diabetes mellitus, and a history of severe pre-eclampsia with HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelet count).
Selection of a Gestational Carrier
Gestational carriers may be known to the intended parents or may be anonymous. Known GCs are typically relatives or friends who volunteer to carry the pregnancy. Anonymous GCs are identified through agencies that specialize in recruiting women to become a GC. The GC should be a minimum of 21 years of age and have delivered a live-born child at term. The use of a GC of advanced age is particularly challenging. The obstetric complication rate, especially the incidence of pregnancy-induced hypertension or gestational diabetes, is much higher. Certainly, evaluation of a woman’s overall health and appropriate screening for underlying medical conditions that might complicate a pregnancy, as well as counseling regarding the obstetric risk, should be performed if considering an older surrogate.
Evaluation of the Intended Parents and Gestational Carrier
The intended parents should undergo a complete medical history and physical examination. Semen analysis should be obtained for the male partner, and an evaluation of ovarian function should be performed for the female partner.
The GC should undergo a complete medical history including a detailed obstetric history, lifestyle history, and physical examination. The GC should have an evaluation of her uterine cavity with hysterosalpingogram, sonohysterogram, or hysteroscopy.
Infectious-disease screening for syphilis, gonorrhea, chlamydia, CMV, HIV, and hepatitis B and C should be performed on the intended parents and the surrogate. The GC also should be screened for immunity to rubella, rubeola, and varicella. In addition, her blood type should be noted.
Counseling of Gestational Carrier and the Intended Parents
Counseling of GCs is intended to provide the GC with a clear understanding of the psychological issues related to pregnancy. With the assistance of a MHP, the gestational surrogate and her partner should explore issues such as managing a relationship with the intended parents, coping with attachment issues to the fetus, and the impact of a GC arrangement on her children and her relationships with her partner, friends, and employers. The intended parents should be counseled regarding their ability to maintain a respectful relationship with the surrogate. The surrogate, the intended parents, and the MHP also should meet to discuss the type of relationship they would like to have. In addition, expectations they have regarding a potential pregnancy should be discussed. This includes a discussion of the number of embryos for transfer, prenatal diagnostic interventions, fetal reduction, and therapeutic abortion, as well as managing the relationship while respecting the carrier’s right to privacy.
LEGAL ISSUES
There are a number of legal issues that concern third-party reproduction. Written consent should be obtained for any procedure. In situations of known sperm or egg donors, both donors, as well as intended parents, are advised to have separate legal counsel and sign a legal contract that defines the financial obligations and rights of the donor with respect to the donated gametes. With embryo donation, in view of the absence of any statute defining the rights and responsibilities of any party involved, it has been suggested that a pre-donation agreement be obtained and a judicial determination of parentage be obtained prior to the donation taking place. With GC arrangements, legal contracts, in addition to delineating financial obligations, may include details regarding the expected behavior of the GC to ensure a healthy pregnancy, prenatal diagnostic tests, and agreements regarding fetal reduction or abortion in the event of multiple pregnancies or the presence of fetal anomalies. Finally, many states allow for a declaration of parentage prior to the child’s birth obviating the need for adoption proceedings. The laws regarding third-party reproduction are either non-existent or different from one state to another. Thus, all couples are advised to consult with an attorney knowledgeable in the area of reproductive law within their individual states.
All potential donors and recipients also should be cautioned that laws may change and anonymity cannot be guaranteed for the future. There are strong movements to eliminate anonymous donation in many countries, and several no longer permit it.
CONCLUSION
The options available through third-party reproduction provide many couples the opportunity to make their dream of parenthood a reality. The comprehensive nature of the screening and counseling of intended parents and their donors or surrogates ensures that the process meets the needs of all involved. Finally, as third-party reproduction is more widely used, there continues to be a broader understanding of the ethical, moral, and legal issues involved. The ultimate goal of physicians, MHPs, and attorneys specializing in reproductive law is to enable this process to move forward as smoothly as possible and bring joy and satisfaction to all parties involved in ensuring the conception and delivery of a healthy child.
